
How does our body turn food into fuel? As a functional nutrition practitioner, this is an ongoing question I consider when helping people support their best health. Why? Well, we need good nutrition to build tissue and run every biochemical function in our body. Specifically, we need minerals, vitamins, amino acids, fatty acids, sugars, fiber, water, and oxygen. Each has its purpose. We affect body function when we compromise good quality raw materials.
Food quality makes a huge difference!
It’s important to eat good quality food in the first place, of course. The best we can source, prepared from whole food ingredients. There’s room for individual difference here. Due to unique metabolic needs, we don’t all thrive on the exact same eating style. That said, a whole foods Mediterranean-style eating plan offers a foundation that helps modulate inflammation, while supporting both cardiovascular health and microbiome balance. Many of us can start from that base and make individual adjustments to suit our personal needs.
But what if you’re already eating a pretty good diet and still have some health issues? This happens frequently. It’s possible to do really well in one area and miss something else. We may underestimate the physiological impact of a big “oops.” Like compromised sleep quality, or an unintended toxin exposure, unmanaged stress, and so on. And when we’re under extra stress, it’s easy to skimp on food quality, despite our best intentions.
The food into fuel process involves Digestion and Distribution of key nutrients
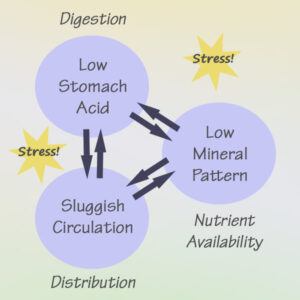
cooperation between the digestive system
and the circulatory system. These functions
are mediated by our response to stress.
These very common factors can impact the two “D’s” of nutrient utilization: Digestion and Distribution. In plain language, we need to be able to break down our food into small particles that can be absorbed into the circulatory system. And then our circulatory system needs to be up to speed (literally) in order to deliver those nutrients to our individual cells.
Any time we have unwanted symptoms present, somewhere behind the scenes there is a breakdown in function. Symptoms as diverse as severe body discomfort, blood sugar regulation issues, mood disturbance, difficulty sleeping, bone health issues, or significant immune failure can all be linked to something as simple and ultimately resolvable as low stomach acid.
Bottom line: Too much of the elements we don’t want [toxins, infection, metabolic waste] coupled with too little of certain elements we do want [nutrients including oxygen, delivered in a timely manner to the cells where they are needed] is a deal-breaker for optimal health. Habitual movement patterns can also keep us stuck in mechanical and energetic disharmony.
Energy production inside our individual cells

This is metabolically where we turn Food into Fuel!
Our bodies largely run on energy that is produced inside the little cellular powerhouses of our individual cells. This process requires good quality materials in proper balance. It’s overly simplistic to think that cellular energy production is the only factor impacting the health of our tissues. It’s not. But it is an important factor, and it’s nutrient-dependent.
“Mitochondrial energy production then powers growth, healing, as well as the complex processes required for adaptation to the changing environment.”
Picard et al. (2018)
In a recent publication on the role of nutrients to enhance cellular energy production after critical illness, a group of researchers from the Netherlands (Wesselink, et al., 2019) identified certain nutrients essential for mitochondrial function. Specifically, their research looked at B Vitamins, ascorbic acid, alpha tocopherol (component of vitamin E), selenium, zinc, coenzyme Q10, caffeine, melatonin, carnitine, nitrate, lipoic acid, and taurine.
It’s interesting that in addition to considering individual nutrients, they also look at the synergistic aspect of combined deficiencies. Cellular energy production is a multi-step process. If we support one particular nutrient while leaving a downstream nutrient deficient, then the outcome may still not be optimal.
Identifying nutrient imbalances
It can be tricky to tease out exactly which nutrients are adequate and which are not. It’s not as simple as running a blood panel or taking a multi-vitamin. There are many considerations and we’re all unique. Many functional practitioners, given this puzzle, look at the person’s symptoms and history in combination with results from a nutrient test (either Hair Tissue Mineral Analysis or blood micronutrient panel), standard lab-ordered blood work, and urinary Organic Acids testing.
Wesselink and colleagues (2019) explain, “Furthermore, it is questionable whether plasma levels of nutrients reflect actual availability in mitochondria. Plasma nutrient levels may be low during critical illness due to increased losses through body fluids and increased permeability of endothelium, redistribution, altered protein binding, and inadequate intake. As a consequence, their plasma levels do not likely reflect tissue storages of micronutrients during critical illness.” (Emphasis added.) Note: the term “endothelium” refers the inner lining of the blood vessels. Inflammation, an inherent factor in critical illness, damages the endothelium.
It is very good to see this research. While there are still many questions, the fact that researchers now consider this type of inquiry worth conducting is quite encouraging!
The essential role of oxygen
Oxygen, specifically, limits the amount of energy produced in any given time and place. Like other nutrients, we must first take oxygen into the body in adequate and consistent quantity. Then our circulatory system must deliver it to the tissues. Most of the energy production inside the cell (Adenosine Triphosphate / ATP) occurs as the result of a series of biochemical steps known as the Electron Transport Chain (ETC), or Respiratory Chain. Picard, et al. (2018) state, “As its name implies, the respiratory chain consumes oxygen.”
Oxygen intake must first be robust. It can be compromised under certain conditions — functional impairment of the lungs, high altitude, sleep disordered breathing, and obstructive face coverings are examples. So first things first. Assess, and then if found lacking, address or consider a supplementary source. Oxygen is not optional.
Systemic or localized disruption of the microcirculation as occurs in a significant inflammatory response, toxin exposure, sympathetic nervous system arousal (stress response), departure from the earth’s electromagnetic field, low nitric oxide state for any other reason, compromises oxygen delivery to the tissues.
Aerobic vs. anaerobic energy production
When oxygen is readily available, the body produces energy through an efficient process known as oxidative phosphorylation (aerobic). If oxygen is limited, then a signaling molecule known as Hypoxic Inducible Factor (HIF) builds up. As our body reaches a threshold of HIF, the cell switches to a different metabolic process known as glycolysis (anaerobic).
The process of glycolysis is not only inefficient, it also creates unfavorable byproducts and changes gene expression in the involved tissues. As such, it’s associated with a number of challenging health conditions characterized by non-resolving inflammation or suppressed immunity — such as rheumatoid arthritis, inflammatory bowel disease, atherosclerosis, and tumors.
How stress interacts with metabolism
Let’s briefly consider stress, and the impact it has on our body’s efforts to convert food into fuel. When faced with a stressor of any sort, we are innately designed to adapt to it. It doesn’t matter what the stress type. Our bodies will begin the adaptation response if we have a challenging day at work, eat a food that doesn’t agree with us, ingest a toxin, have a short night of sleep, get exposed to a “bug,” speak or think about ourselves unkindly, set a new personal best for the 50 yard dash, or any host of other life events.
What does it mean to adapt? Adaptation calls for a variety of basic and complex functions.

to produce “heat energy” for basic operation, communicate
needs or shortages, create substances such as
hormones and neurotransmitters, and remove waste.
The healthier we are to begin with, the better we adapt.
- We may need to think of a new solution.
- Remember how to find our way home.
- Run from a predator or dangerous situation.
- Cool our bodies when they’re overheated or warm them up if we’re exposed to frigid conditions.
- Laser-focus our eyesight on one particular point or scan the broadness of the whole landscape.
- Move extra oxygen-carrying air quickly through our lungs.
- Inactivate a stealth “bug” that hitched a ride on our salad greens.
- Remove metabolic waste and other unneeded material from our bodies.
- Form a clot to stop bleeding.
- Heal tissue.
- Grow new cells.
- Instruct our cells how to survive in the absence of enough of a needed element. Say oxygen, water, or certain nutrient minerals.
- Stop an infection.
- Perceive the difference between safety and danger.
- Inspire hope in a friend or loved one.
- And so much more.
Adapting to stress requires a lot of energy!
As we are actively adapting to stress, our energy demands increase. As Picard et al. (2018) state, “Even though, in fact, basic life-sustaining biological functions also require energy for their maintenance, the energy requirement for stress responses is above the basal needs of the organisms; hence, the emphasis here on the link between stress and energy.”
Sometimes, we may have too much stress to handle at once, or over a period of time. As this happens, our bodies are no longer able to adapt successfully. Our ability to turn food into fuel is inadequate. We begin to experience disease or metabolic dysregulation. This can be a complex situation that involves trouble keeping blood sugar balanced, significant mood issues, cognitive issues, disruptions to our sleep / wake cycles, fatigue, cardiovascular disease, formation of tumors, joint and tissue degeneration, immune-mediated challenges, premature aging and so on.
This is a brief introduction to a rather involved topic. We’ll return to it again in future posts. Optimizing adaptation to stress is the essence of functional health care, whether it comes from a nutritional perspective or a medical one. The strategy I take in my functional nutrition consulting is, generally, to decrease stress inputs while we balance nutrients, enhance nutrient delivery, build microbiome health, and explore certain lifestyle factors like mindset and sleep quality. Ultimately, our intent is to become more adaptable and resilient.
Next steps — turning food into fuel
In coming posts, we will explore both Digestion and Distribution of nutrients separately, along with some guidance on the approaches that can make a difference toward enhancing these critical functions. We’ll also be taking a deeper dive into the functional effects of Stress on both these systems. If you would like to explore personal guidance in applying these strategies to your own situation, check out my Functional Health Consulting page, where you will find contact information. I would be happy to hear from you!
Resources:
- Cummins, E. P., Keogh, C. E., Crean, D., & Taylor, C. T. (2016). The role of HIF in immunity and inflammation. Molecular aspects of medicine, 47-48, 24–34. https://doi.org/10.1016/j.mam.2015.12.004
- Ghosh, T. S., Rampelli, S., Jeffery, I. B., Santoro, A., Neto, M., Capri, M., Giampieri, E., Jennings, A., Candela, M., Turr oni, S., Zoetendal, E. G., Hermes, G., Elodie, C., Meunier, N., Brugere, C. M., Pujos-Guillot, E., Berendsen, A. M., De Groot, L., Feskins, E., Kaluza, J., … O’Toole, P. W. (2020). Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut, 69(7), 1218–1228. https://doi.org/10.1136/gutjnl-2019-319654
- https://www.niddk.nih.gov/health-information/digestive-diseases/digestive-system-how-it-works
- Jennings, A., Berendsen, A. M., de Groot, L., Feskens, E., Brzozowska, A., Sicinska, E., Pietruszka, B., Meunier, N., Caumon, E., Malpuech-Brugère, C., Santoro, A., Ostan, R., Franceschi, C., Gillings, R., O’ Neill, C. M., Fairweather-Tait, S. J., Minihane, A. M., & Cassidy, A. (2019). Mediterranean-Style Diet Improves Systolic Blood Pressure and Arterial Stiffness in Older Adults. Hypertension (Dallas, Tex. : 1979), 73(3), 578–586. https://doi.org/10.1161/HYPERTENSIONAHA.118.12259
- Lee, C. C., Wu, C. Y., & Yang, H. Y. (2020). Discoveries of how cells sense oxygen win the 2019 Nobel Prize in Physiology or medicine. Biomedical journal, 43(5), 434–437. https://doi.org/10.1016/j.bj.2020.05.019
Resources, continued:
- McGettrick, A. F., & O’Neill, L. (2020). The Role of HIF in Immunity and Inflammation. Cell metabolism, 32(4), 524–536. https://doi.org/10.1016/j.cmet.2020.08.002
- Picard, M., McEwen, B. S., Epel, E. S., & Sandi, C. (2018). An energetic view of stress: Focus on mitochondria. Frontiers in neuroendocrinology, 49, 72–85. https://doi.org/10.1016/j.yfrne.2018.01.001
- Trayhurn P. (2019). Oxygen-A Critical, but Overlooked, Nutrient. Frontiers in nutrition, 6, 10. https://doi.org/10.3389/fnut.2019.00010
- Tsigalou, C., Konstantinidis, T., Paraschaki, A., Stavropoulou, E., Voidarou, C., & Bezirtzoglou, E. (2020). Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines, 8(7), 201. https://doi.org/10.3390/biomedicines8070201
- Wesselink, E., Koekkoek, W., Grefte, S., Witkamp, R. F., & van Zanten, A. (2019). Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clinical nutrition (Edinburgh, Scotland), 38(3), 982–995. https://doi.org/10.1016/j.clnu.2018.08.032
- https://en.wikipedia.org/wiki/Metabolism Accessed 3/27/2021


















